
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.
1 brazing
The brazing properties of carbon steel and low alloy steel depend to a large extent on the type of oxide formed on the surface of the material. As the temperature increases, four types of oxides of Y-Fe2O3, a-Fe2O3, Fe3O4, and FeO are formed on the surface of the carbon steel. These oxides are porous and unstable except for Fe3O4, which are easily removed by the flux and are easily reduced by the reducing gas, so that the carbon steel has excellent brazing properties.
For low alloy steels, if the alloying elements contained are relatively low, the oxide present on the surface of the material is essentially an oxide of iron, and the low alloy steel at this time has the same brazing properties as carbon steel. If the alloying elements contained are increased, especially the addition of elements such as A1 and Cr which tend to form stable oxides, the brazing property of the low-alloy steel may be deteriorated. In this case, a more active flux or dew point should be used. Low shielding gas is brazed.
2 brazing material
(1) Brazing of brazing carbon steel and low alloy steel includes soldering and brazing. The most widely used solder in soldering is tin-lead solder. The wettability of this solder to steel increases with the increase of tin content. Therefore, solder with high tin content should be used for the sealing joint. The tin-steel in the tin-lead solder may form a Fe-Sn intermetallic compound layer at the interface. To avoid the formation of the layer compound, the brazing temperature and the holding time should be properly controlled. The shear strength of several typical tin-lead brazed brazed carbon steel joints is shown in Table 1. Among them, brazed joints with w(Sn) of 50% braze have the highest joint strength and do not contain antimony. The joint strength of the weld is higher than that of the niobium.
When brazing carbon steel and low alloy steel, pure copper, copper zinc and silver copper zinc brazing filler metal are mainly used. Pure copper has a high melting point and is easy to oxidize the base metal during brazing. It is mainly used for gas shielded brazing and vacuum brazing. However, it should be noted that the gap of the brazed joint should be less than 0.05 mm, so as to avoid the problem that the joint gap cannot be filled due to the good fluidity of the copper. The carbon steel and low alloy steel joints brazed with pure copper have high strength, the general shear strength is in the range of 150 to 215 MPa, and the tensile strength is distributed between 170 and 340 MPa.
Table 1 Shear strength of carbon steel joints welded by tin-lead solder
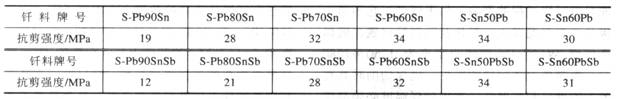
Table 2 Strength of low carbon steel joint brazed by silver copper and zinc

Compared with pure copper, the copper-zinc solder reduces the melting point of the solder due to the addition of Zn. In order to prevent evaporation of Zn during brazing, a small amount of Si may be added to the copper-zinc solder on the one hand, and rapid heating methods such as flame brazing, induction brazing, and dip soldering may be employed on the other hand. Both carbon steel and low alloy steel joints brazed with copper-zinc solder have good strength and ductility. For example, the carbon steel joint brazed with B-Cu62Zn brazing material has a tensile strength of 420 MPa and a shear strength of 290 MPa. The melting point of the silver-copper-zinc solder is lower than the melting point of the copper-zinc solder, which facilitates the brazing operation. This solder is suitable for flame brazing, induction brazing and furnace brazing of carbon steel and low alloy steel, but the amount of Zn should be reduced as much as possible in the furnace brazing, and the heating rate should be increased. The joints of carbon steel and low alloy steel are welded with silver-copper needle solder to obtain joints with good strength and plasticity. The specific data are listed in Table 2.
(2) Flux or shielding gas is required for flux brazing of carbon steel and low alloy steel. The flux is often determined by the solder and brazing method chosen. When a tin-lead solder is used, a mixture of zinc chloride and ammonium chloride may be used as a flux or other special brazing. The residue of this flux is generally highly corrosive and should be carefully cleaned after brazing.
When brazing with copper-zinc solder, FB301 or FB302 flux should be used (see JB/T6045-92 flux for brazing, the same below), ie borax or a mixture of borax and boric acid: in flame brazing A mixture of methyl borate and formic acid may also be used as the flux, wherein B2O3 vapor is used for the membrane removal.
When silver-zinc-zinc solder is used, FBl02, FBl03 and 1FB104 fluxes, ie a mixture of borax, boric acid and certain fluorides, may be selected. The residue of this flux has a certain degree of corrosiveness and should be removed after brazing.
3 brazing technology
The surface to be welded is cleaned mechanically or chemically to ensure that the oxide film and organic matter are completely removed. The surface after cleaning should not be too rough and should not be attached to metal scraps or other contaminants.
Brazing of carbon steel and low alloy steel can be carried out by various common brazing methods. For flame brazing, a neutral or slightly reducing flame should be used. The flame should be directly heated to avoid solder and flux. Rapid heating methods such as induction brazing and dip brazing are very suitable for brazing of quenched and tempered steel, and brazing should be selected at a quenching or tempering temperature to prevent the base material from softening. When brazing low-alloy high-strength steel in a protective atmosphere, not only the purity of the gas is required, but also the gas flux must be used to ensure the wetting and spreading of the brazing filler metal on the surface of the base metal.
The residue of the flux can be removed by chemical or mechanical means. The residue of the organic flux can be wiped or cleaned with an organic solvent such as gasoline, alcohol or acetone: the residue of a highly corrosive flux such as zinc chloride and ammonium chloride should be neutralized in an aqueous NaOH solution, followed by hot water and cold water. Cleaning; the residue of boric acid and borate flux is not easily removed and can only be solved by mechanical means or by boiling in boiling water for a long time.
January 13, 2024
January 13, 2024
Contactar proveedor
January 13, 2024
January 13, 2024

Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.

Fill in more information so that we can get in touch with you faster
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.